product description
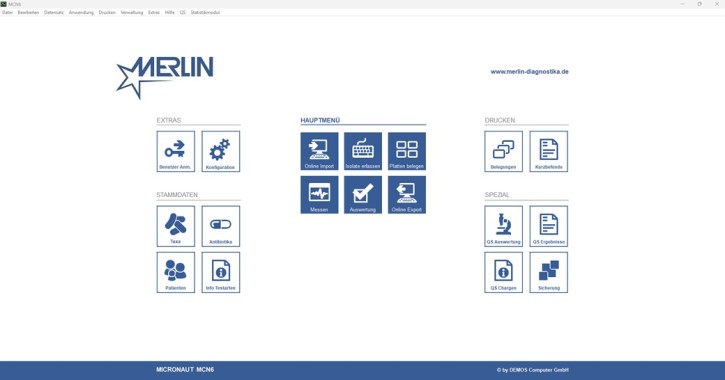
MICRONAUT software UR MCN6
for an EUCAST and RiliBÄK-compliant evaluation within the framework of the MICRONAUT system
• Unique patient ID (import from PVS or free entry)
• Definition of the test type (e.g. UR base plate, extended antibiogram)
• Regular updates based on EUCAST via a simple update
Current version: EUCAST 14.0
• Creation of work order lists
• Control of the photometric measurement after 18-24 hours
• Free or supported evaluation and validation with the integrated expert system to check the resistance determination for plausibility and effectiveness
• Printout of findings or export (via LDT/GDT) to the PVS
• Local data backup (on PC, local storage medium or server storage location)
Incl. QA module for quality assurance for internal quality controls (reference bacteria)
• Evaluation of the reference bacteria according to the current EUCAST specification
• Documentation and Deviation management directly in the software
Incl. statistics module for statistical evaluation of the result data of the identification or resistance results of the MICRONAUT-UR system.
With just a few clicks, self-defined statistics can be carried out or predefined evaluations can be called up, e.g.
• Simple germ statistics/pathogen statistics
• Germ statistics over a defined period of time (month, quarter, year)
• RIS statistics
The reports can be generated as a PDF file or in Excel format and save you the need to keep separate, manual lists or similar.
In Germany, the MPBetreibV prescribes training for medical devices. Training is subject to a fee and is not included in the price.
Please contact us for further information and a quote by email or using our contact form.
We regularly offer training courses for working with the MICRONAUT system, such as MICRONAUTEN CLUB training courses or various AUROSAN eLearning courses. You can find further information and current dates in our training overview.